

Ceftriaxone Sodium CAS 104376-79-6
- Product Name :
Ceftriaxone Sodium CAS 104376-79-6
- CAS No. :104376-79-6
 Contact us
Contact us

Product Details
| CEFTRIAXONE SODIUM |
| Chemical Name | Ceftriaxone Sodium |
| CAS Number | 104376-79-6 |
| Molecular Formula | C18H21N8NaO8S3 |
| Molecular Weight | 596.58 |
| Molecular Structure | 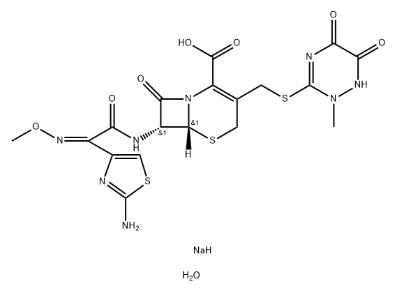 |
| Storage | Keep in dark place,Inert atmosphere,2-8°C |
| Application | Antibiotics, APIs. Generally used for the treatment of respiratory tract infection, urinary system infection, gonorrhea and preoperative infection prevention. |
| SPECIFICATION |
| ITEMS | SPECIFICATION |
| Appearance | Almost white or yellowish, slightly hygroscopic, crystalline powder |
| Solubility | Freely soluble in water, sparing soluble in methanol, very slightly soluble in anhydrous ethanol |
| Specific Optical Rotation | -155°~170° |
| Identification | Meets the requirements |
| pH | 6.0~8.0 |
| Appearance of Solution | The solution is clear |
| Not more intensely coloured than reference solution Y5 or BY5 |
| Related Substances | Other unknown single impurity≤1.0% |
| Total impurities≤ 4.0% |
| Residual Solvents | Ethanol≤0.5% |
| Acetone≤0.5% |
| Dichloromethane≤0.06% |
| Methanol≤0.3% |
| Water | 8.0%~11.0% |
| Visible Particles | Meets the requirements |
| Particulate Matter | ≥10μm, ≤3000/g |
| ≥35μm, ≤300/g |
| Bacterial Endotoxins | ≤0.08EU/mg |
| Sterility | Meets the requirements |
| Assay | 96.0%~102.0% of Ceftriaxone Sodium
(C18H16N8Na2O7S3), calculated on anhydrous basis |
| Standard | USP, EP10.0, ChP |


Immediately Consult
Tag:GMP,USP,Antibiotics,APIs

Other Products

New Products

 Contact us
Contact us

 Tel: +86-519-8661-9955
Tel: +86-519-8661-9955  Email: info@comwin-china.com
Email: info@comwin-china.com  Fax: +86-519-8661-3190
Fax: +86-519-8661-3190 ADD:24th Floor, Jiaye International Commercial Plaza 99 Yanling West Road, Changzhou Jiangsu 213003 China
ADD:24th Floor, Jiaye International Commercial Plaza 99 Yanling West Road, Changzhou Jiangsu 213003 China


